COVID-19 Vaccines

COVID-19 vaccine news is being released on a daily basis and can be difficult to keep up with. In this blog, we’ll answer your questions regarding COVID-19 vaccine safety, eligibility, benefits, side effects, the history and science behind mRNA, and scheduling opportunities in Central Florida.
Pfizer Vaccine Update: As of May 12, 2021, the CDC has approved the use of the Pfizer vaccine for ages 12 years and older. Learn more from the CDC here.
Johnson & Johnson Vaccine: As of April 25, 2021, the CDC and FDA recommend use of Johnson & Johnson’s COVID-19 vaccine resume in the United States, after a temporary pause. Learn more from the CDC here.
Fully Vaccinated Patients: If it’s been more than two weeks since you’ve completed your COVID-19 vaccine (including second doses for Pfizer and Moderna), please review CDC guidelines for what you can do when you’ve been fully vaccinated.
For the Latest Updates: Please visit our Patient Advisory page for the latest information on COVID-19.
Are COVID-19 Vaccines Safe?
Yes. Certain groups may have an elevated chance of adverse reactions, specifically women under 50 who are pregnant, on oral contraceptives, with thrombocytopenia (low platelet count), taking hormone therapies. We encourage you to schedule an appointment with your clinician before getting the Johnson & Johnson vaccine. The FDA granted Emergency Use Authorization to the Pfizer-BioNTech COVID-19 vaccine for those 16 and older, the Moderna COVID-19 vaccine for ages 18 and older, and the Johnson & Johnson vaccine for ages 18 and older after rigorous clinical testing and evaluation that included tens of thousands of participants. “Whenever FDA approves or authorizes a vaccine, our work doesn’t stop there,” says Dr. Stephen Hahn, commissioner of the U.S. Food and Drug Administration (FDA).
![]() COVID-19 vaccines must adhere to specific FDA guidelines for safety and efficacy, and the FDA also sets up ongoing surveillance programs that continue after clinical trials. You can also learn more about how the CDC has expanded their safety monitoring system to ensure the safety of the COVID-19 vaccine, including the V-safe after vaccination health checker and the Vaccine Adverse Event Reporting System (VAERS). It takes collaboration from health and safety agencies around the United States and the world to continue to review and ensure vaccine safety.
COVID-19 vaccines must adhere to specific FDA guidelines for safety and efficacy, and the FDA also sets up ongoing surveillance programs that continue after clinical trials. You can also learn more about how the CDC has expanded their safety monitoring system to ensure the safety of the COVID-19 vaccine, including the V-safe after vaccination health checker and the Vaccine Adverse Event Reporting System (VAERS). It takes collaboration from health and safety agencies around the United States and the world to continue to review and ensure vaccine safety.
Who Is Eligible for the COVID-19 Vaccine?
As of Monday, April 5th, all Florida residents will be eligible for the COVID-19 vaccine. Please note that age restrictions (16+ or 18+) may vary by vaccine type. Patients can visit the state of Florida vaccine site locator and pre-register now. Patients can also register at the Federal website FLVAX and visit vaccinefinder.org for the latest on vaccine inventory. Please visit our scheduling resources section on our patient advisory for more information.
What Are the Benefits of the COVID-19 Vaccine?
Available vaccines have been shown to be highly effective at preventing COVID-19. Clinical trials resulted in a 95% effective rate for Pfizer, a 94.1% effectiveness for Moderna, and 85% effective for Johnson & Johnson. While the vaccines have been shown to be effective at preventing COVID-19 symptoms in those vaccinated, there is not enough evidence at this time to determine whether they prevent transmission of COVID-19 to another person.
 Without enough evidence on transmission, it is vital that we continue following CDC guidelines, including social distancing and wearing a mask in public places. Peak vaccine effectiveness occurs a few weeks after receiving your vaccines, as your body continues to build immunity. In addition to preventing COVID-19, the vaccines may also help keep you from getting seriously ill if you do contract COVID-19. The CDC recommends the vaccine even for those who have already been infected by COVID-19.
Without enough evidence on transmission, it is vital that we continue following CDC guidelines, including social distancing and wearing a mask in public places. Peak vaccine effectiveness occurs a few weeks after receiving your vaccines, as your body continues to build immunity. In addition to preventing COVID-19, the vaccines may also help keep you from getting seriously ill if you do contract COVID-19. The CDC recommends the vaccine even for those who have already been infected by COVID-19.
Talk to your Primary Care Provider for more information on your specific situation and recommended vaccine guidance by scheduling a TeleHealth Appointment today.
Are There Any Side Effects to the COVID-19 Vaccine?
The most common side effects for the Pfizer, Moderna and Johnson & Johnson vaccines include pain at the infection site, fatigue, headache, fever, chills and muscle pain. Side effects can last up to several days and usually resolve on their own.
 According to AdventHealth, “These coronavirus vaccine side effects are temporary, and shouldn’t discourage anyone from getting this potentially life-saving immunization.” Side effects for Pfizer and Moderna are more likely to be experienced after the second dose. These side effects, such as fever, are considered normal signs that the body is building immunity. As we mentioned, the FDA and CDC continue to monitor vaccination efforts across the US for any severe side effects or adverse reactions. You cannot get COVID-19 from the vaccine.
According to AdventHealth, “These coronavirus vaccine side effects are temporary, and shouldn’t discourage anyone from getting this potentially life-saving immunization.” Side effects for Pfizer and Moderna are more likely to be experienced after the second dose. These side effects, such as fever, are considered normal signs that the body is building immunity. As we mentioned, the FDA and CDC continue to monitor vaccination efforts across the US for any severe side effects or adverse reactions. You cannot get COVID-19 from the vaccine.
What are mRNA Vaccines?
The goal of a vaccine is to teach our immune system what a virus looks like and how to develop protection against future infection. Vaccines have been studied, developed, and distributed for hundreds of years, and have led to the eradication of small pox as well as the sharp decline of polio, tetanus, influenza (the flu), measles, and more.

Vaccines rely on antigens, usually in the form of an inactivated (killed) or attenuated (weakened) virus, which help trigger a response in our immune system by imitating an infection.
Vaccines that use messenger RNA, commonly referred to as mRNA, follow the same scientific principles of traditional vaccines in order to teach our body how to produce antibodies. So what’s the difference? Rather than containing a version of the virus, COVID-19 mRNA vaccines give instructions to our cells to make a harmless piece of what is called the “spike protein.” The spike protein is found on the surface of the virus that causes COVID-19 and triggers an immune response leading to the production of antibodies.
Once we’ve produced enough antibodies, our body can protect us against future infection. Think of the mRNA vaccine like an instruction guide on protecting against COVID-19, without the risk of contracting the actual virus. Messenger RNA never enters the nucleus of the cell, and therefore does not affect or interact with our DNA in any way.
How Long Has mRNA Been Around?
The discovery of messenger RNA took place in the 1960s. In 2005, mRNA science experienced a breakthrough, which is being used in today’s COVID-19 vaccines. “When we started this, we weren’t thinking about curing pandemics,” says Dr. Weissman. “We were thinking about making new vaccines… Even before COVID hit, we had already set up clinical trials for mRNA vaccines.”
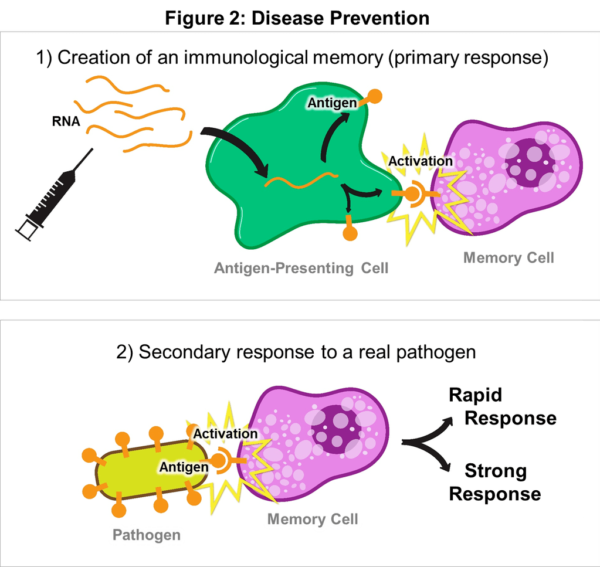 Messenger RNA has been studied for decades by the scientific and academic communities. Consider this quote from a Harvard Medical School blog published in 2015, called RNA Vaccines: A Novel Technology to Prevent and Treat Disease.
Messenger RNA has been studied for decades by the scientific and academic communities. Consider this quote from a Harvard Medical School blog published in 2015, called RNA Vaccines: A Novel Technology to Prevent and Treat Disease.
“The production of RNA-based vaccines is more rapid compared to production of traditional vaccines. This rapid production could be a major advantage in face of sudden pandemics.”
Dr. Anthony Komaroff, Editor in Chief of the Harvard Health Letter, writes “Like every breakthrough, the science behind the mRNA vaccine builds on many previous breakthroughs.” From understanding the structure of DNA and mRNA to genetic sequencing of viruses to triggering immune response and more, the development of COVID-19 mRNA vaccines relies upon decades of scientific studies and advancements from around the world.
Where Can I Get the COVID-19 Vaccine?
COVID-19 vaccination efforts are underway across Central Florida and the nation. Southwest Orlando Family Medicine endorses all eligible patients to receive any version of the COVID-19 vaccine currently available. For answers to common questions about COVID-19, please visit our COVID-19 FAQ blog.
We maintain a hopeful outlook on global efforts to combat the virus as COVID-19 vaccine development and distribution continues. We ask for patience and understanding as we continue to strongly recommend following COVID-19 safety guidelines. Thank you for entrusting Southwest Orlando Family Medicine with keeping you and your loved ones healthy.
—
Disclaimer: All information presented on this website is intended for educational purposes only and not intended to replace your individual medical advice. Please review this information with your clinical team to ensure it is appropriate for your individual medical needs. The information contained is not intended to diagnose, treat, cure or prevent any disease.
